Healthcare Professionals
A Foundation of Clinical Evidence
The most clinically studied atrial shunt in heart failure patients with EF≥40%.
Over 1,000 Corvia Atrial Shunt patients have been studied to date through multiple studies around the world. Clinical results have consistently established the safety of the Corvia Atrial Shunt System and have demonstrated encouraging efficacy in heart failure patients with an EF≥40% and elevated left atrial pressure.1-4
>675
Corvia Atrial Shunts
implanted globally
>2300
Implanted patient-years of follow-up4
10+
Years post-implant for the longest ongoing Corvia Atrial Shunt patients4
30+
Peer-reviewed publications
on the Corvia Atrial Shunt performance & mechanism of action
3-Year Responder Group Results From REDUCE LAP-HF II
REDUCE LAP-HF II is the largest device therapy trial for heart failure patients with EF≥40%. The study discovered a specific demographic of heart failure patients who respond positively to atrial shunt therapy, termed “Responders”.
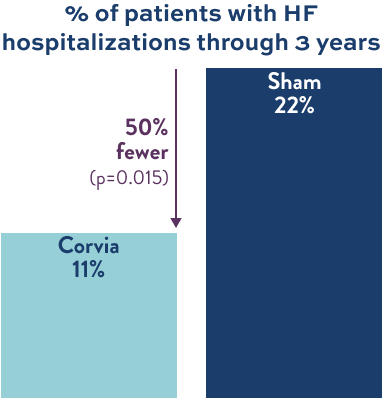
Now with follow-up data spanning three years, Responders with the Corvia Atrial Shunt continue to experience significantly fewer HF events and an improved quality of life compared to sham control.
3-YEAR HIGHLIGHTS1,3
Performance vs sham*
50%
Fewer patients hospitalized
>10 point
Greater improvement in KCCQ
72%
More Corvia Atrial Shunt patients improved ≥1 NYHA Class
Safe
No statistically significant differences in key safety endpoints
REDUCED HF EVENTS1
Consistent HF event reduction through 3 years
IMPROVED QUALITY OF LIFE1,3,5
Corvia patients experienced large and consistent improvement across all KCCQ domains.
90%
More Corvia Atrial Shunt patients experienced a >20 point KCCQ-OSS improvement by year 3 vs sham patients
NNT = 5
Treating just 5 patients with a Corvia Atrial Shunt yields an additional patient with a large (>20-point) 3-year KCCQ improvement
70%
greater chance of improving by one or more NYHA classes compared to sham at 3 years
EXCELLENT EASE OF USE & SAFETY1, 2, 4
PROCEDURAL SUCCESS
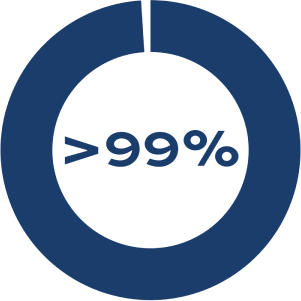
Successful shunt implantation when transseptal puncture is performed for shunt placement
PATENCY
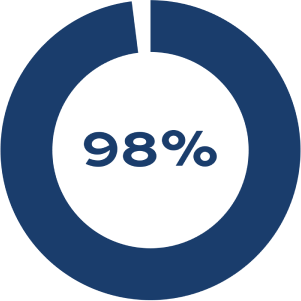
Flow detected with no echocardiographic evidence of thrombus through 2 years of follow-up
KEY SAFETY OUTCOMES
No statistically significant differences in CV mortality, stroke, or other serious adverse events vs sham.
| Events in Responders Through 3 Years | Corvia Atrial Shunt (N=161) | Sham Control (N=152) | p-value |
|---|---|---|---|
| Cardiovascular mortality | 4.4% | 2.2% | 0.35 |
| Non-fatal ischemic stroke | 1.9% | 0.0% | 0.25 |
| Thrombo-embolic complications (TIA) | 0.0% | 1.5% | 0.21 |
| New or worsening kidney dysfunction | 10.7% | 19.3% | 0.04 |
| Newly acquired persistent or permanent atrial fibrillation or flutter | 5.7% | 6.7% | 0.72 |
| Myocardial infarction | 2.2% | 2.5% | 1.00 |
HEAR FROM THE EXPERTS
Global experts Prof. Maja Cikes and Prof. Finn Gustafsson share insights and their perspectives on the 2-year results from the REDUCE LAP-HF II trial.
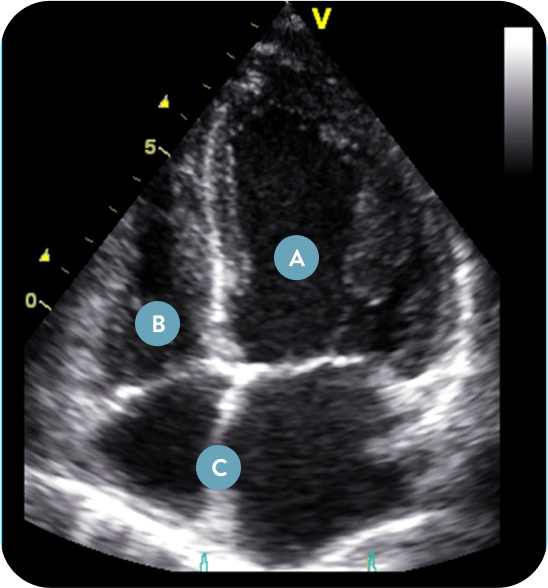
ECHOCARDIOGRAPHIC EVIDENCE OF LEFT HEART UNLOADING2
Recent 2-year echocardiographic data from REDUCE LAP-HF II provides evidence of the favorable long-term effect of atrial shunting on heart structure and function, and support the definition of Corvia Atrial Shunt “Responders”. The analysis showed that the Corvia Atrial Shunt led to reverse remodeling of the left heart chambers and increases in volume of the right heart chambers, without compromising right ventricle systolic function compared to sham.
| CONCLUSION | ECHOGRAPHIC EVIDENCE |
|---|---|
 Shunt is unloading left heart | Decreased LA/LV volume vs sham Decreased LA pressure vs sham Decreased mitral regurgitation grade vs sham |
 Shunt is not negatively affecting right heart | No difference in PASP vs sham No difference in RA systolic pressure vs sham No difference in RV systolic function vs sham |
 Blood flow through shunt from LA to RA | Increased RA volume vs sham Increased RV end-diastolic volume vs sham |
- Litwin, Sheldon E., et al. “Long term safety and outcomes after atrial shunting for heart failure with preserved or mildly reduced ejection fraction: 5-year and 3-year follow-up in the REDUCE LAP-HF I and II trials.” American Heart Journal 278 (2024): 106-116.
- Patel, R., et al. “Atrial Shunt Device Effects on Cardiac Structure and Function in Heart Failure With Preserved Ejection Fraction: The REDUCE LAP-HF II Randomized Clinical Trial.” JAMA Card. (2024)
- Statistical analyses conducted by Baim Institute for Clinical Research. Data on file.
- Unpublished data as of March 1, 2025 compiled from Corvia Clinical Trials and on file at Corvia Medical.
- All subjects blinded to treatment arm for two years.


