CORVIA® ATRIAL SHUNT SYSTEM
A Novel Treatment for Symptomatic Heart Failure
The only direct treatment for high left atrial pressures (LAP)

The Corvia Atrial Shunt is a novel, minimally invasive cardiac implant for patients suffering from Heart Failure with Preserved Ejection Fraction (HFpEF) or Mildly Reduced Ejection Fraction (HFmrEF). Designed to reduce elevated LAP, the primary contributor to HF symptoms, the Corvia Atrial Shunt offers the most advanced therapeutic option for HF patients with an EF≥40%.
The Corvia Atrial Shunt is the most widely studied interatrial shunt for heart failure. It has been implanted in over 675 patients worldwide, and more than 250 patients have had the shunt for over 5 years.1
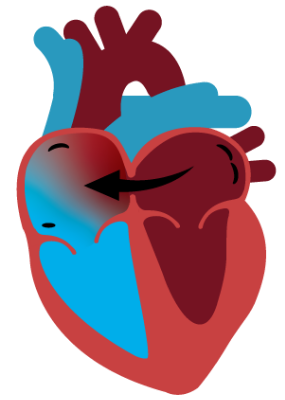
Dynamic Decompression
Atrial shunting safely relieves the high pressures that cause HF symptoms by connecting the two upper chambers of the heart. This connection allows the heart to dynamically decompress by directing blood from the left to right atrium on demand, thereby reducing HF symptoms.
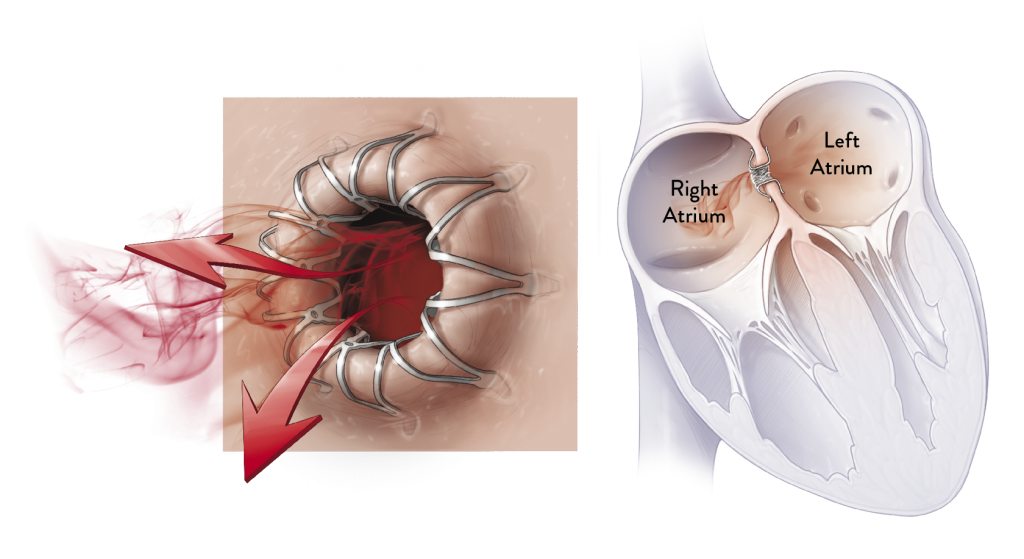
HOW IT WORKS
The Corvia Atrial Shunt is implanted via a minimally invasive procedure. During this procedure, an interventional cardiologist or electrophysiologist inserts a catheter (small tube) in a vein near the groin to access the heart. This catheter is then used to create a very small passage in the heart wall between the right and left atria where the shunt is placed. The newly created passage allows blood to flow from the high pressure left atrium to the lower pressure right atrium. As a result, the pressure in the left side of the heart and the lungs decreases, and heart failure symptoms are reduced.
WATCH HOW IT WORKS
By facilitating continuous and dynamic decompression of the left atrium, the Corvia Atrial Shunt is intended to reduce heart failure hospitalizations and improve heart failure symptoms and quality of life.2
MAGNETIC RESONANCE (MR) IMAGING INFORMATION

Non-clinical testing demonstrated that the Corvia Atrial Shunt is MR Conditional. A patient with this device can be scanned safely in an MR system immediately after placement under the following conditions:
- Static magnetic field of 1.5 tesla and 3 tesla, only
- Maximum spatial gradient magnetic field of 4,000 gauss/cm (extrapolated) or less
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 watts/kg for 15 minutes of scanning (i.e., per pulse sequence) in the normal operating mode of operation for the MR system
- Under the scan conditions defined, the Corvia Atrial Shunt is expected to produce a maximum temperature rise of 2.4°C after 15 minutes of continuous scanning (i.e., per pulse sequence).
In non-clinical testing, the image artifact caused by the Corvia Atrial Shunt extends approximately 5 mm from this device when imaged using a gradient echo pulse sequence and a 3 tesla MR system.
- Unpublished data as of March 1, 2025 compiled from Corvia Clinical Trials and on file at Corvia Medical.
- Gustafsson, F, Petrie, M, Komtebedde, J. et al. 2-Year Outcomes of an Atrial Shunt Device in HFpEF/HFmrEF: Results From REDUCE LAP-HF II. J Am Coll Cardiol HF. 1 Jun 2024. https://doi.org/10.1016/j.jchf.2024.04.011
