Healthcare Professionals
REDUCE LAP-HF II Trial
LANDMARK TRIAL in HFpEF
REDUCE LAP-HF II is the largest device therapy trial for heart failure patients with preserved ejection fraction (HFpEF), the biggest unmet need in cardiology. This global, landmark, sham-controlled trial is evaluating the Corvia Atrial Shunt in heart failure patients to reduce HF-related hospitalizations and improve quality of life through a reduction in left atrial pressure (LAP), the primary cause of HF symptoms.
BACKGROUND
REDUCE LAP-HF II primary endpoint analyses demonstrated the Corvia Atrial Shunt was safe, significantly reduced HF events, and improved quality of life in a newly defined optimal patient population for atrial shunting (‘responder’ population) compared to sham control at one year.1
2-Year Responder Group Highlights
50%
Reduction in HF event rate compared to sham control
46%
Greater improvement in KCCQ compared to sham control
98%
Patency
Based on evaluable echo studies
STUDY POPULATION
Randomized, double-blind, placebo-controlled 621 patients
PATIENTS INCLUDED: ≥40 years with symptomatic HF
and EF ≥40% with elevated exercise PCWP (≥25 mm Hg)
and left-to-right gradient (≥5 mm Hg)
RESPONDER COHORT Identified (n=313)
Patients without significant pulmonary vascular disease (PVR<1.74 WU) and no pacemaker
Follow-Up Blinding Maintained Through 2 Years
CONCLUSION
The Corvia Atrial Shunt improved quality of life and reduced heart failure (HF) symptoms and events through 2 years without a safety signal in appropriately selected HF patients with an ejection fraction (EF) ≥40%.2
WHY IT MATTERS
This is the first long-term, randomized data of atrial shunt performance in heart failure patients. It demonstrates the positive effect of the atrial shunt on reducing HF events and improving health status persists through 2 years in ‘responders’.
2-Year Responder Group Outcomes (N=313)2
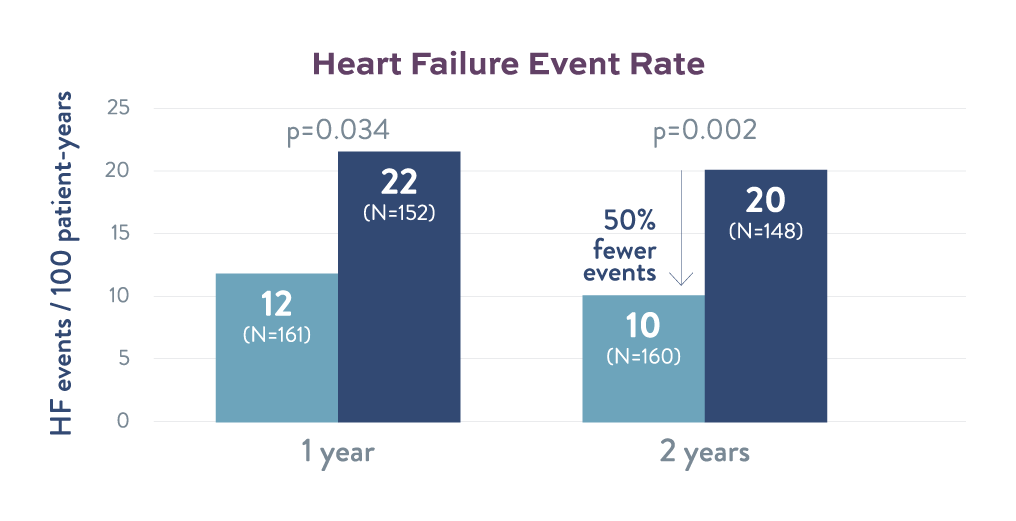
Heart Failure Event Rate
Corvia Atrial Shunt therapy led to a 50% reduction in the rate of heart failure events
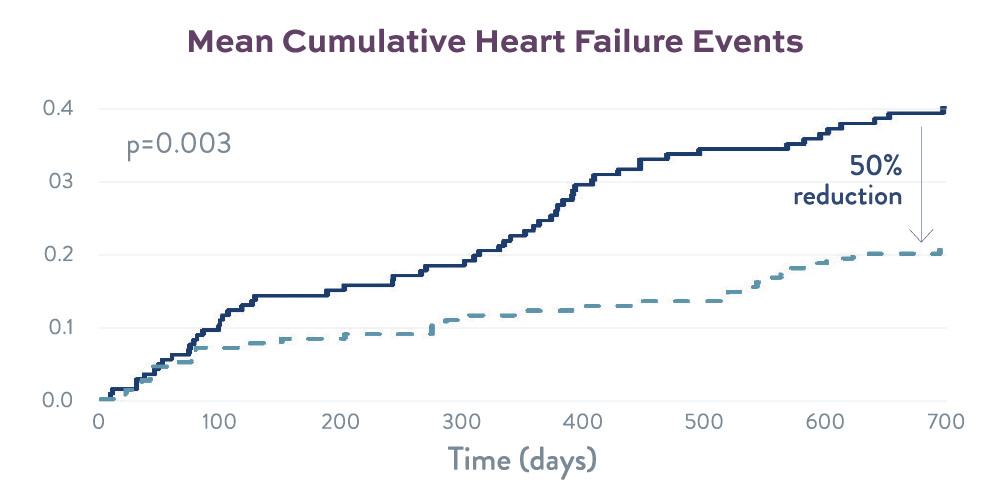
Cumulative Heart Failure Events
Significant separation of heart failure event curves at the 2-year mark.
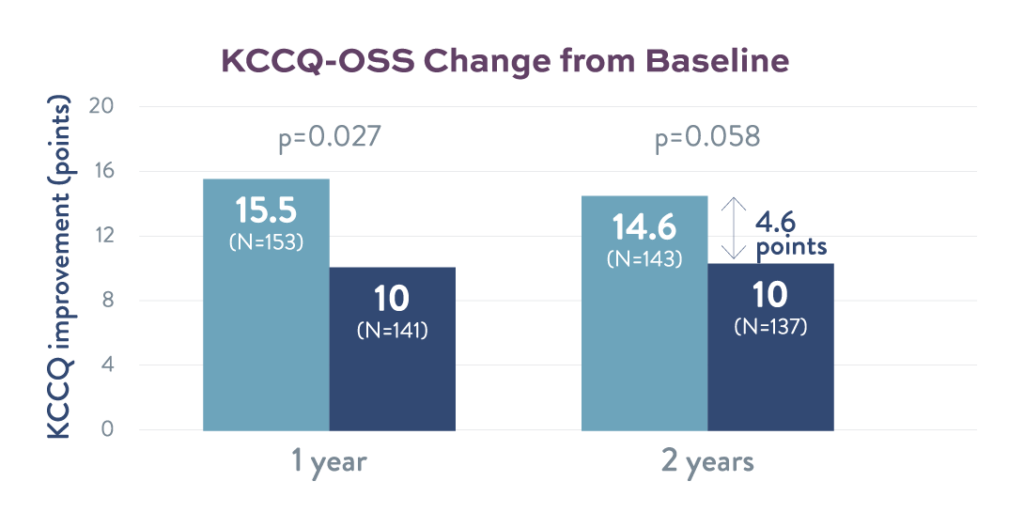
Quality of Life
At 2 years, atrial shunt patients reported a 46% greater improvement over their baseline KCCQ score compared to sham control
Key Safety Outcomes
| Events in Responders through 2 years | Corvia Atrial Shunt (N=161) | Sham control (N=152) | P-value |
|---|---|---|---|
| Cardiovascular mortality | 3/160 (1.9%) | 2/148 (1.4%) | 0.72 |
| Non-fatal ischemic stroke | 3/160 (1.9%) | 0/148 (0.0%) | — |
| New or worsening kidney dysfunction | 14/160 (8.8%) | 22/148 (14.9%) | 0.10 |
| Major adverse cardiac events | 6/160 (3.8%) | 4/148 (2.7%) | 0.61 |
| Other thrombo-embolic complications | 1/160 (0.6%) | 1/148 (0.7%) | 0.96 |
| ≥30% Decrease in TAPSE | 5/160 (3.1%) | 4/148 (2.7%) | 0.83 |
HEAR FROM THE EXPERTS
Global experts Prof. Maja Cikes and Prof. Finn Gustafsson share insights and their perspectives on the 2-year results from the REDUCE LAP-HF II trial.


Circulation Publication
Read the REDUCE LAP-HF II Responder publication in Circulation
2-Year Clinical Summary
Download REDUCE LAP HF-II 2-Year Clinical Summary
Interested in learning more?
Please reach out to your local Corvia field representative or contact us.
- Borlaug, BA, Blair, J, Bergmann, MW et al. Latent Pulmonary Vascular Disease May Alter the Response to Therapeutic Atrial Shunt Device in Heart Failure. Circulation. 2022;10.1161. (in press)
- Gustafsson, F, Petrie, M, Komtebedde, J, et al. Two-Year Outcomes of an Atrial Shunt Device in HFpEF/HFmrEF: Results from REDUCE LAP-HF II. Jnl Amer Coll Cardiol HF (under review).